High-Affinity TCR
Most T cells express alpha-beta TCRs, composed of disulfide-bonded alpha and beta chains. A small subset of T cells express gamma-delta TCRs composed of disulfide-bonded gamma and delta chains.
An alpha-beta TCR on the surface of a T cell binds directly to an antigenic peptide in the context of human leukocyte antigen (HLA) on the surface of a cell. Hence, TCR genes, a promising targeting reagent, allow you to redirect T cells and target drugs to cells of a particular specificity.
Increasing T cell avidity has been correlated with improved anti-tumor activity. However, naturally occurring TCRs are typically of low affinity and can be modified to increase their affinity by altering amino acid composition of the antigen-binding domain of the alpha and beta chains, or by allogeneic approach. As a targeting reagent, such as bi-specific T cell engager, high-affinity TCR is an essential requirement.
AgainChance has established a platform technology to efficiently hunt a high-affinity alpha-beta TCR gene specific to a peptide antigen displayed by HLA-A0201.
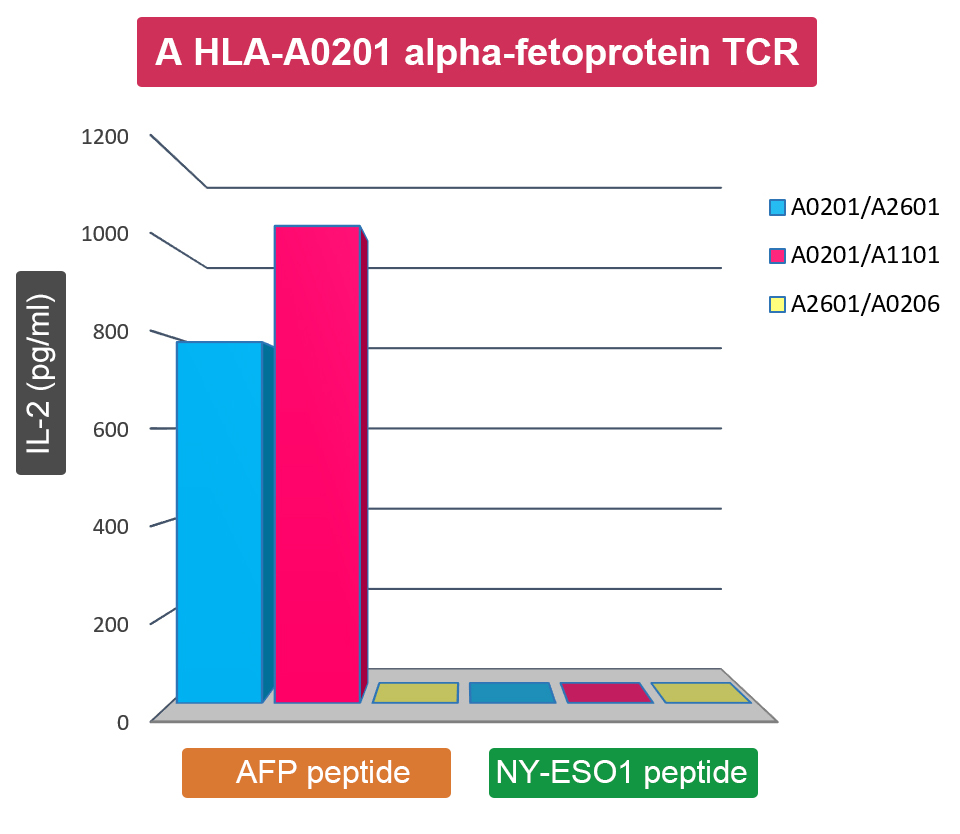
HLA-restricted recognition of endogenously expressed alpha-fetoprotein (AFP) by TCR-transduced Jurkat cells. The TCR gene isolated from an AFP specific CD8+ T cell clone was transduced into Jurkat cells. TCR-transduced cells were cocultured with a panel of AFP or NY-ESO-1 gene-transfected dendritic cells (DCs) (genetically typed as A0201/A2601, A0201/A1101 and A0201/A2601, respectively). The antigen reactivity and HLA restriction of transduced Jurkat cells were measured in an interleukin-2 (IL-2) release assay by ELISA. Untransduced Jurkat cells did not release IL-2 when stimulated with AFP/NY-ESO-1 gene-transfected DCs.